
|
|||||||||||||||
|
| |||||||||||||||
Spring diatom blooms follow upwelling events  Many spring algal blooms on the Central Coast involve chain-forming diatoms such as Skeletonema costatum. Although these algae are large for diatoms, they are still invisible to the naked eye (the white line is as about long as a human hair is wide). (Source: Phytopedia) The gusty northwest winds of March often cause the first spring upwelling events. These upwelling events bring nitrate and other nutrients toward the surface and act like fertilizer, sparking the growth of tiny microscopic algae called diatoms. Like plants on land, diatoms grow by capturing energy from the sun and adsorbing water, nutrients, and carbon dioxide from their surroundings. However, unlike most land plants, diatoms are single-celled organisms. Given plenty of sunlight and nutrients, they don't grow larger, but split in half, essentially cloning themselves. Under optimum conditions, some diatoms can divide (and thus double their population) every twelve hours. A little math will show you that this can yield a lot of diatoms in a very short time. The optimum growing conditions for diatoms are similar to those for land plants--they like to have plenty of sunlight, water, and nutrients. Living in the ocean, diatoms have no shortage of water. So the two factors that control the timing and extent of phytoplankton blooms are sunlight and nutrients. During the winter months, both sunlight and nutrients are in short supply. There isn't much sunlight because the days are short and often cloudy. When the sun does shine, it is at such a low angle that most of the light bounces off the sea surface and does not penetrate into the water below. To make matters worse, winter storm waves and winds from the southeast churn the ocean, mixing water from the surface down to depths of 500 feet or more. This constant churning often carries diatoms down to depths where sunlight cannot penetrate and they cannot grow. Unless they get lucky and are carried back up into the sunlit waters within a day or two, most diatoms cannot survive this treatment, and will either die or go dormant, forming seed-like cysts, which sink down toward the sea floor. Given sunlight and a stable water column, however, diatoms typically begin to bloom within one or two days after northwest winds bring a pulse of nutrients to the surface. Stronger winds bring up deeper water that contains higher concentrations of nutrients. Winds of longer duration bring up a larger volume of deep water and spread it farther across the ocean surface. Thus, stronger, more sustained northwest winds often result in denser, more extensive diatom blooms. However, if the winds last long enough, they may generate currents that carry these diatoms far from the coast. In addition to supplying nutrients, upwelling events may also help trigger diatom blooms by bringing dormant diatom cysts up from the sea floor into the sunlit surface waters, where they may come to life again. In fact, many diatom species that bloom in early spring have cysts that spend the winter on or near the sea floor, just waiting to be carried up to the surface by upwelling events. Upwelling favors fast-growing diatoms Now let's take a closer look at the star players in these blooms--the diatoms themselves. On a year-round basis, diatoms are by far the most abundant type of phytoplankton found off Central California. One marine biologist counted over 200 different species of phytoplankton in Monterey Bay, of which almost two thirds were diatoms. Almost all of the diatoms that bloom in spring are cylindrical in shape and are part of a group known as "centric diatoms." 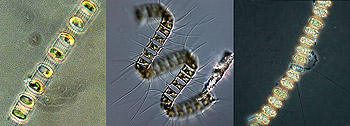 Many spring algal blooms on the Central Coast involve large, chain-forming diatoms such as (right to left) Skeletonema costatum, Chaetoceros debilis, and Thalassiosira aestivalis (Sources: NOAA/Seagrant, Plankton Net, UCSC/CIMT) Centric diatoms require plenty of sunlight and have a variety of adaptations that help them gather this precious resource. If you zoom in on a centric diatom's “shell” under the microscope, you will see that its surface is etched in beautiful, ornate patterns. Marine biologists have speculated that some of these surface features act like miniature lenses to focus light on the parts of the diatom that convert sunlight into energy. Diatoms in Monterey Bay seem to grow best within about 100 feet of the sea surface. If they stay right at the surface, they may get "sunburned." If they sink too deep, they may not get enough sunlight to survive. Larger diatoms, which sink relatively quickly, are especially favored by strong upwelling events, which enhance vertical mixing and help keep them near the surface. Some diatoms have long spines that create drag, so that they don't sink out of the sunlit surface water too quickly. Many centric diatoms link up in chains, which sink even more slowly than individual diatoms (and are also harder for small animals to eat). Healthy, well-nourished diatoms can also increase their buoyancy by storing oil within their bodies (just as we store our excess food as fat). However, if they run out of nutrients, these diatoms must tap into their oil reserves and start to sink. This may explain why entire populations of diatoms sometimes sink down from the surface waters after using all up of the available nutrients. Small populations of centric diatoms live in Central Coast waters all year long, waiting for just the right conditions that will allow them to bloom. Each upwelling event and subsequent phytoplankton bloom tends to be dominated by a single species of diatom. Like spring wildflowers, different species of diatoms often bloom in succession at specific times of year. 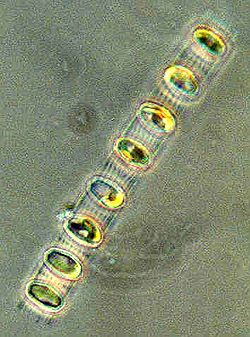 A chain of Skeletonema costatum diatoms under a microscope. (Source: NOAA/Seagrant) The diatom most characteristic of the beginning of upwelling is Skeletonema costatum, which typically blooms between February and April. Like another early blooming diatom, Thalassiosira aestivalis, Skeletonema diatoms spend the winter as dormant cysts on the sea floor. These cysts are carried up into the water column by the first strong upwelling event of the year. They also have an affinity for iron and may respond rapidly to the pulse of iron that enters surface waters during the first major upwelling event of the year. Note: As upwelling continues through the spring, the near-bottom turbid layer is swept clear and less iron is brought to the surface, allowing different types of algae to bloom. By late summer or fall, very little iron is available, and blooms of centric diatoms become much less frequent. The third group of diatoms to bloom in the spring succession are members of the genus Chaetoceros. Different species of Chaetoceros bloom during different months. For example Chaetoceros debilis is most likely to bloom in March or April, while Chaetoceros venheurckii and Chaetoceros radicans are more common in May and June, respectively (and will be discussed in later chapters).  Chaetoceros debilis is one of the main diatoms that bloom in spring along the Central Coast> (Source: Plankton Net) Chaetoceros diatoms account for the vast majority of the phytoplankton that bloom during spring on the Central Coast. They are also common at other times of year. In fact, they are probably the most abundant type of diatom in the entire Eastern Pacific Ocean. At least eighteen different species of Chaetoceros have been found in Monterey Bay alone. What most centric diatoms do well, Chaetoceros diatoms do best--that is, sucking up nutrients and reproducing very quickly when nutrients are plentiful, then forming large stocks of resting spores that sink down from the surface to blanket the sea floor. Chaetoceros diatoms are so good at sucking up nutrients that scientists have seen both nitrate and carbon dioxide virtually disappear from surface waters within one to two days of a Chaetoceros bloom. In looking at the spring succession of diatoms, it is interesting to note that smaller diatoms such as Skeletonema are more likely to appear early in the season, while larger species such as Chaetoceros tend to bloom later in spring. This seasonal size shift may occur because small diatoms are better at staying near the surface, collecting sunlight, or taking advantage of the less frequent upwelling events of early spring. Conversely, the frequent upwelling and active wind-driven mixing that occurs later in spring may favor large diatoms. Blooming diatoms provide essential food for tiny marine animals such as copepods, for larger swimming animals such as anchovies and krill, for gelatinous filter feeders such as salps, and for bottom-dwelling filter-feeders such as mussels, oysters, scallops, and clams. Many of these animals rely on the abundant and more or less predictable food supplied by spring phytoplankton blooms. In fact, as we shall see, the life cycles of many marine animals are timed to coincide with the food provided by different types of diatoms that bloom throughout the spring. Microscopic eggs and larvae drift around coastal waters In the Introduction to this month, I describe my observations of some of the tiny animals (zooplankton) that drift and swim in the surface waters of Monterey Bay during March. This section focuses on the zooplankton that look like science-fiction aliens--the tiny larvae of fish, crabs, sea stars, barnacles, and other animals that live (as adults) in the tide pools and kelp beds. Many of these near-shore animals release eggs or larvae that spend anywhere from several days to several months drifting around in the open ocean. Such eggs and larvae are especially abundant in Monterey Bay during February and March. During their time in the plankton, larval fish and invertebrates feast on spring-bloom diatoms, as well as on each other. If they survive, the larvae eventually develop into juveniles and (in some cases) begin to look more like their parents. At this point, the young animals must somehow find their way back from the open ocean to the kelp beds, intertidal areas, or the sea floor. If they are unable to return to the coast or to unable find suitable habitat, these young, rapidly-developing animals will die. These larvae face many challenges as they drift on the currents during the spring upwelling season. But many animals have found ways to benefit from this lifestyle as well. For details on this process, see "The hard lives of young ocean drifters". Anchovies release eggs Anchovy spawn all year long, but those living along the coast from Monterey Bay down to Southern California do most of their spawning between February and April of each year. Sometimes large schools of adults spawn just offshore of estuaries such as Elkhorn Slough. This may allow the incoming tide to carry their eggs into the warm, protected waters of the estuary, where the young will grow more rapidly and perhaps face fewer predators. Other anchovies spawn in the relatively warm, protected coastal waters south of Point Conception. Female anchovies spawn repeatedly, releasing batches of over 100,000 eggs about every 10 days or so. The eggs hatch within a few days. The tiny larvae (about 1/4 inch long) live off their yolk for a week or two, and then begin capturing young copepods and tiny microscopic algae called dinoflagellates. When they first begin feeding, anchovy larvae in the open ocean rise upward from deeper water until they reach a depth where there are lots of young copepods and/or algae. Once they start feeding, their bodies adjust to the density of the water around them, so that they stay at this one depth or at least within water of this density. Most of the time, this helps them stay where the food is. During some years, late storms stir up the water column, moving the larvae to depths where there is no food. Since anchovy larvae cannot swim upward or re-adjust their buoyancy, they may starve to death. For this reason, there are huge variations in the number of anchovy larvae that survive from year to year. Rockfish larvae dominate the spring zooplankton Although anchovies and lampfish form huge schools in Central Coast waters, rockfish larvae are the most common type of fish larvae that marine biologists find drifting around Central Coast waters during March. In addition to providing food for a variety of birds and marine mammals, rockfish are also a very popular food item for humans. Most people have seen rockfish in grocery stories, where they are usually marketed as “red snapper.” In fact, over a dozen different types of fish are sold as "red snapper" in California. Even the experts sometimes have a hard time telling them apart. This is not too surprising considering that perhaps 65 different species of rockfish live along the Central California Coast and hybrids abound. Most Central California rockfish mate between November and January. Females typically brood their fertilized eggs within their bodies for several months after mating. Some time between January and July (depending on the species) these eggs hatch within the female's body and are released into the water as tiny, swimming larvae, perhaps 1/16 to 1/8 inch long.  Newly hatched rockfish larvae (about 1/16-inch long). (Source: David Csepp, NMFS/NOAA) Depending on the age and type of rockfish, each female releases between 100,000 and 2,000,000 larvae in a single massive birthing event. These clouds of larvae are often released at twilight or after dark, perhaps to reduce the chances of their being eaten by visual predators. In coastal areas where many rockfish reside, winds and seas may also be calmer at night. The tiny larval rockfish do not have the yolk sacs that serve as “bag lunches” for some fish larvae. However, rockfish larvae are born with very well-developed eyes and mouths, which “allow immediate feeding on live food” as one researcher delicately put it. Fortunately for us, these hundreds of millions of hungry, well-developed mouths consume only small prey, particularly other larval fish. Most larval rockfish are visual predators, primarily feeding in the daytime, and staying within about 300 feet of the sea surface. They typically drift or swim in the surface waters for several months before settling down to the sea bottom. During their time in the plankton, they must hunt and eat almost continuously in order to survive. Actually, the vast majority of them don’t survive, which is why so many are released in the first place. Three to eight weeks after being released into the plankton, the larval rockfish develop into juveniles. At this point, their bodies are still transparent and only an inch or two long. These juvenile rockfish are essential prey for thousands of seabirds that feed and nest along the Central Coast during the summer. Because different species of rockfish release larvae during different months, several “waves” of rockfish larvae move through the plankton between February and June. In the following paragraphs, we'll look at what's happening to the rockfish in each of these waves during March. The first wave - rockfishes that settle down to the sea floor in March The first wave of winter-spawning rockfish larvae to settle the seafloor include two common species, vermilion and canary rockfish. These larvae were released between November and January, and have been drifting and swimming in the open ocean since then. Most of these larvae settle down to the sea floor in February or early March, before upwelling really gets going. This reduces the chances that the young rockfish will be carried away from shore by upwelling currents.  A vermilion rockfish (Source: US EPA) Vermilion rockfish (Sebastes miniatus) get a particularly early start, with larvae that turn into juveniles and settle down to the sea floor in February, before the upwelling season even begins. Although adult vermilion rockfish live in deep water, their juveniles settle down near shore in water that is less than 100 feet deep. The young Vermilion rockfish settle down wherever hard surfaces such as rocks, worm colonies, surf-grass beds, or pier pilings stick up through a sandy bottom. At this point, the juvenile vermilion rockfish are mottled brown in color. This provides good camouflage, because they often hide in red algae, which are relatively abundant in early spring. Like many other deep-water fishes, as the young vermilion rockfish mature, they gradually become orange-red in color and migrate out across the continental shelf to depths of several hundred feet. Deep water rockfish are often orange or red because this color makes them invisible in the deep sea, where red light from the sun never penetrates. Vermilion rockfish are one of the most common species typically sold as “red snapper.” Another rockfish species in the “first wave” are Canary rockfish (Sebastes pinniger), which settle down to the sea floor soon after the first upwelling event of the spring--slightly later than vermilion rockfish. In fact, their settling appears to be triggered by a drop in water temperature or some other environmental change that coincides with the beginning of upwelling.  Young-of-the-year canary rockfish, about three inches long. (Source: Ammann/NOAA/SIMON) Like vermilion rockfish, young canary rockfish settle down to the sea floor just offshore of the kelp beds, at depths of about 60 to 80 feet, congregating in rocky areas surrounded by sand. Hiding in the shelter of the rocks during the daytime, the newly settled juveniles venture out at night to hunt over the nearby sand flats. Canary rockfish, like vermilion rockfish, gradually migrate into deeper water as they get older, eventually ending up on the deeper parts of the continental shelf. They may also migrate parallel to the coast, colonizing areas up to several hundred miles from where they first settled down. Adult canary rockfish are often found near steep undersea cliffs and pinnacles, where they hunt small fish and krill. Krill congregate in huge swarms in such areas, particularly along the edges of Monterey Submarine Canyon. During late spring and summer, canary rockfish apparently gorge on these swarms of krill, putting on weight that will help them survive the leaner seasons of fall and winter. The second wave - rockfishes whose larvae are drifting in the plankton during March The second and largest wave of rockfish larvae includes some species that release young in winter (November to January), as well as others that are released later, between January and March. This second wave includes many common kelp-bed rockfish, including blue rockfish, olive rockfish, and black rockfish, as well as deeper species such as blackgill, copper, and yellowtail rockfish. Although not in the rockfish family, two other predatory, bottom-dwelling fish--cabezon and lingcod, also release larvae in January or February. By March, these swarms of second-wave fish larvae are already a month old and are busy devouring other larvae, as well as copepods. Rockfish larvae in this second wave must be well adapted to upwelling, since many of them need to return to coastal areas to settle down in April and May, at the height of the upwelling season. In fact, as mentioned previously, some rockfish in this group, including blue rockfish and olive rockfish, are more abundant during years when upwelling is strong.  A school of blue rockfish swim around a kelp stipe. (Source: Chad King, NOAA/SIMON) Blue rockfish and olive rockfish are common schooling fish of the Central-Coast kelp beds. Blue rockfish (Sebastes mystinus) have a particularly long larval period that may last as long as four to five months (depending on who you talk to). During this time, the young fish may be carried dozens or even hundreds of miles from shore, and survive by eating copepods and krill as well as other planktonic animals. Despite the fact that these larvae are often carried offshore by ocean currents, come April or May, massive swarms of juvenile blue rockfish often appear in the kelp beds. How they make their way back to shore is still a matter of speculation. Note: Other species of rockfish don’t even get around to releasing larvae until the middle of the upwelling season. These spring spawners release a “third wave” of rockfish larvae later in spring and covered in the section on open ocean life in May. Larvae of predatory fish prey on other drifters The practice of releasing larvae before upwelling begins (and thus having well-developed larvae in the plankton just as upwelling starts) must provide some significant advantages for bottom-dwelling predators, because it is not unique to rockfish. At least two other common predatory fish, cabezon and lingcod, have similar habits. Cabezon larvae  Cabezon on seafloor. (Source: John Ugoretz, California Department of Fish and Game)  Cabezon eggs surrounded by California hydrocoral (Stylaster californicus) and strawberry anemones(Corynactis californica). (Source: John Ugoretz, California Department of Fish and Game) Cabezon (Scorpaenichthys marmoratus) spawn from November through January, laying eggs on the sea floor. These eggs typically hatch in February. The newly hatched cabezon larvae are about one quarter inch long, with a large yolk sac (which acts like a bag lunch for the young fish). By March, the cabezon larvae have grown to about 3/8-inch long, and have used up their yolk. At this time, they become voracious predators on other plankton, incessantly gobbling copepods, crab larvae, barnacle larvae, fish eggs, and fish larvae, all of which are plentiful in March. Cabezon larvae seem to have a particular fondness for eggs and larvae of their own kind. In fact, cannibalism may account for a significant proportion of the cabezon larvae that die during their larval stage. Cabezon larvae are frequently found dozens of miles offshore, along with rockfish larvae. Unlike most rockfish larvae, however, cabezon larvae are only observed at the sea surface at night. Presumably they spend the days in deeper waters, and come up to the surface to feed, following their prey, some of which (such as Dungeness crab larvae) also spend their days in deeper water and migrate up toward the surface each night. Cabezon spend a total of three to four months in the plankton, first as larvae, then as small, silvery juveniles an inch or two long. Like blue rockfish, juvenile cabezon somehow find a way to migrate back to the kelp beds between April and June, during the height of the upwelling season. It may help that by this time, the juvenile cabezon have grown to about 2 1/2 inches long and are very active swimmers. Since they are vertical migrators, it is also possible that deep, subsurface currents help carry them back toward shore. Lingcod larvae The larvae of another formidable kelp-bed predator, the lingcod (Ophiodon elongatus) also stalk their fellow drifters during March. Lingcod lay their eggs in shallow rocky areas in October or November, and the eggs typically hatch in January or February, just before upwelling starts. After the eggs hatch, the 1/4-inch-long larvae spend about ten days hanging out near the seafloor and living off of their yolk sacs. When the "bag lunch" of their yolks is all used up, the lingcod larvae rise up toward the ocean surface and drift around in the ocean for several months. During this time, they fatten up on copepods and their eggs, as well as on amphipods, crab larvae, and young krill. They begin moving back toward shore in March, when they are two to three inches long. But they won't actually settle down to the nearshore seafloor until May or June. |
