
|
|||||||||||||||
|
| |||||||||||||||
|
Spring diatom blooms Ocean grazers Calanoid copepods Krill larvae Copepods and krill larvae Anchovies and hake Winter-spawning rockfish Dungeness crab larvae King salmon Deep-sea midwater animals Seafloor animals English sole and halibut |
Spring diatom blooms fuel Central Coast food webs  Chaetoceros debilis is one of the main diatoms that bloom in spring along the Central Coast> (Source: Plankton Net) As upwelling events become more frequent in April, each pulse of upwelled water provides fertilizer for untold trillions of diatoms. These conditions favor fast-growing diatoms, which take advantage of the abundant nutrients (especially nitrate and iron) dissolved in the upwelled water. One species of diatom, Chaetoceros debilis, seems to be particularly well adapted to blooming at the height of the upwelling season. This large, chain-forming diatom thrives when strong upwelling events are followed by brief periods of "relaxation" (when the northwest winds die down). The cold, nutrient-rich water of April also favors blooms of a few other diatoms in the genus Chaetoceros, including Chaetoceros venheurckii. Many of these diatoms are found in cold waters of the Eastern Pacific as far north as Alaska. Note: Over the entire spring-bloom season (March through June), a succession of different diatom species may dominate the bloom during repeated upwelling events, with each species reaching peak abundance for roughly a month. These diatom species (in order of succession) include:
All of these Chaetoceros diatoms are relatively large. When supplied with plenty of nutrients, they reproduce very rapidly, their populations doubling during each successive day of a bloom. Under these conditions, they also tend to form long "daisy-chains." Ocean grazers feast on spring-bloom diatoms The long chains of spring diatoms provide a more concentrated food source than do smaller individual diatoms and other micro-algae that bloom later in the year. Thus, April and May is the prime feeding and growing season for ocean grazers. Such grazers include the tiny shrimp-like creatures called copepods, young krill, the microscopic drifting larvae of intertidal animals, and even small fish such as anchovies and sardines. The microscopic, drifting animals of the open ocean are called zooplankton. In early spring (February and March) zooplankton populations within five or ten miles of the coast are dominated by fish eggs (especially anchovy eggs) and larvae of nearshore animals (especially crabs, including hermit crabs and Cancer crabs). By April, however, Central Coast zooplankton populations are changing rapidly. Many crab larvae, after drifting for several months at sea, are starting to turn into young crabs, and will soon sink down to their adult habitats on the sea floor, in kelp beds, or in tide pools. Other drifting larvae, such as those of winter-spawning rockfish, have become juveniles, but will spend another month in surface waters before sinking toward the seafloor. As fish eggs and larvae become less abundant in the nearshore zooplankton, a new crop of tiny, drifting animals appears. Many of these are diatom eaters. Their young hatch in early spring and develop just in time to take advantage of the late spring peak in diatoms. Two of the most important of these diatom eaters are copepods and krill larvae. Calanoid copepods bloom  A calanoid copepod in the genus Neocalanus, a cold-water species similar to those found on the Central Coast. (Source: NOAA) April often sees massive blooms of copepods in Monterey Bay and the Gulf of the Farallones. In such sheltered areas, copepod populations increase rapidly during March and April and typically peak in late April or early May. Note: Farther from shore, over the continental shelf, copepod populations may not reach peak abundance until June or even July. Many types of copepods eat nothing but diatoms. These copepods have long bristles and comb-like feeding appendages that they sweep through the water to gather diatoms, like a combine harvesting wheat. Other copepods are omnivores, eating both diatoms and other microscopic animals in the water column. Many of the diatom-eating copepods of spring are calanoid copepods in the genera Calanus, Eucalanus, Nannocalanus, and Rhincalanus. Like the diatoms on which they feed, calanoid copepods live in Central Coast waters all year long, but they don't reproduce rapidly until after the spring diatom blooms begin. Calanoid copepods are relatively large as zooplankton go--the biggest ones are about the same size and shape as the commas on this page. Just as the spring-bloom diatoms are larger than many algae that bloom later in the year, so the copepods that bloom in spring are perhaps ten times longer than (and have 100 times the volume of) the smaller copepod species that are common in fall or winter. Calanoid copepods may also have more oils than other, smaller species of copepods. Thus, calanoid copepods provide a highly concentrated food source for larger predators, such as anchovies. Some copepods spend most of the day in deep water--below 200 feet--where upwelling currents cannot carry them offshore and visual predators cannot see well. These copepods can tell when diatoms near the surface are blooming because such blooms change the color of the sunlight filtering down through the surface waters. If they are hungry enough, the copepods in deep water will swim up toward the surface until they reach the depth at which the diatoms are blooming (diatom blooms are often concentrated in well-defined layers). When the copepods reach a layer of blooming diatoms, they swim horizontally, gorging themselves on the diatoms. Many calanoid copepods store food in the form of oil droplets within their bodies. When diatoms are scarce, these copepods can live off their oil droplets for weeks or even months, until the next upwelling event and resulting diatom bloom provides a new supply of food. In contrast, many smaller species of copepods can only survive for a few days without food, and are only found in large numbers immediately following diatom blooms. Krill larvae eat diatoms and grow rapidly While all these copepods are feeding and reproducing within five or ten miles of the coast, other diatom grazers become abundant farther from shore, at or beyond the edge of the continental shelf. Most people know these small, shrimp-like animals by their Inuit name, krill; marine biologists call them euphausiids (pronounced "you-fow-sids"). 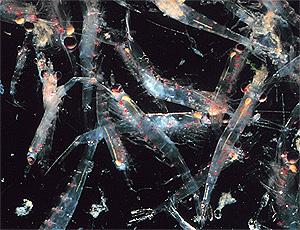 A swarm of krill in the water off the Farallon Islands (Source: NOAA) Like calanoid copepods, krill cannot reproduce rapidly enough to send out a new flock of young in response to every diatom bloom. However, most krill follow a fairly predictable yearly cycle. Microscopic krill larvae are typically born in early spring, mature in summer, mate in fall, and then release eggs and die in winter. By April or May, the krill larvae that were born in early spring have grown large enough to begin feeding on spring-bloom diatoms. Note: When conditions are favorable, a second yearly generation of krill may hatch in summer; these krill will mature and reproduce in late fall or even in winter. Like most animals, krill grow faster and are more likely to survive when they have plenty of food. Thus, krill are more abundant during years when upwelling events are frequent and strong and diatoms are plentiful in offshore waters. Conversely, during years when upwelling is weak or inconsistent, many krill larvae starve to death. So do many fishes and birds that depend on krill as food. Note: Newly hatched krill larvae tend to stay within about 300 feet of the ocean surface--where the diatoms are. Because they live near the sea surface, young krill larvae may be carried far from shore by upwelling currents. As they mature, however, the krill migrate down to deeper water and congregate along the slopes of submarine canyons and at the edge of the continental shelf. Copepods and krill larvae provide food for myriads of other animals Copepods and krill larvae provide food for many larger marine animals on the Central Coast. In the following paragraphs, we will look at a few of these predators--anchovies and juvenile rockfish near the sea surface; hake and lanternfish in the deep sea; and Dungeness crab larvae and king salmon in nearshore waters. Note: A number of these "first-order" predators (anchovies, juvenile rockfish, etc.) are eaten by larger predators such as salmon, seabirds, and marine mammals. These larger animals rely on smaller prey to provide a predictable food source for themselves and their young each year. Humans also eat many first-order predators such as anchovies and rockfish, which support some of the largest commercial fisheries on the California Coast. Anchovies and hake arrive in large schools Starting in March or April, schools of anchovies and hake migrate northward up the California coast. Many of these are young fish that were born off Southern California from February through April. Others are born in nearshore waters or estuaries along the Central California coast. The young anchovies and hake eat diatoms and other microscopic algae. As the anchovies grow up, they begin eating calanoid copepods and other large zooplankton. As the hake get older, they switch to eating krill and krill larvae. Winter-spawning rockfish reach juvenile stage By April, the rockfish larvae that were born in January or February have become one- to three-inch-long juveniles. Over the next couple of months, these juvenile rockfish will settle down to new lives on the seafloor or in the kelp beds. In preparation for this transition, their transparent bodies (appropriate for life in the midwater) are becoming opaque and pigmented. Like young anchovies and hake, juvenile rockfish rely on calanoid copepods and krill larvae for food. Juvenile rockfish, in turn, provide essential food for many surface-diving seabirds, which congregate and begin nesting in April. Dungeness crab larvae become juveniles  Megalops larvae (the last larval stage) of the Dungeness crab - within a few weeks, these larvae will settle down to the seafloor to become juvenile crabs. (Source: Oregon Department of Fish and Wildlife) Among the rapidly growing crab larvae drifting around the coastal waters in April are larvae of Dungeness crab (Cancer magister). Since January, these larvae have been drifting around the offshore waters, eating copepods and diatoms. Despite the strong upwelling currents in April, the larvae typically congregate near shore, in the upwelling shadow area just outside the Golden Gate. By April, the Dungeness crab larvae have grown to about the size of a dime. They look somewhat like adult crabs, but have transparent bodies, big bulging eyes, and little tails that help them swim. They mostly hunt other zooplankton, but also eat spring-bloom diatoms. Like winter-spawning rockfish, these Dungeness crab larvae can be very numerous near the ocean surface, and provide essential food for many animals from seabirds to salmon. The megalops larvae will begin settling down to the seafloor in May or June. Note: Along the Oregon coast, Dungeness crab megalops larvae appear later than on the Central California Coast, in late spring or early summer (sometimes in large numbers). Catches of adult Dungeness crabs vary dramatically from year to year, depending on how strong upwelling was two or three years previously, when the crabs were larvae. King salmon hunt juvenile Dungeness crab Before they settle down to the seafloor, these Dungeness crab larvae provide essential food for another animal that is a delicacy to humans - the king salmon. In spring, thousands of king salmon gather in the Gulf of the Farallones to fatten up before swimming inland to spawn in the Sacramento River Delta in the fall. In early April, these salmon feed primarily on one type of animal--the late-stage larvae of Dungeness crabs. Since the Dungeness crab larvae are so small and the salmon are so large (up to 48 inches long), the salmon must eat a lot of larvae. Over 7,000 Dungeness crab larvae have been found in the stomach of a single king salmon (and some lucky grad student got to count them all). In fact, during years when salmon are plentiful, Dungeness crab larvae often become scarce in nearshore waters. In late April, as the Dungeness crab larvae begin to sink to the sea floor, king salmon switch to eating juvenile krill. They seem to prefer a relatively large species of krill--Thysanoessa spinifera. These juvenile krill congregate in vast swarms offshore of the Golden Gate in April and May. Note: Many krill are pink in color. Wild salmon that eat these krill absorb this pink pigment, giving their flesh a characteristic "salmon" color. The flesh of farmed salmon, on the other hand, is typically gray unless artificial coloring or krill extract is added to their salmon chow. April is also the month when the salmon-fishing season typically opens on the Central Coast. Although the salmon season typically runs from April through October, many recreational fishermen seem to believe that the best fishing is on the first day of the season. At harbors from Monterey to San Francisco, you can see them lining up at the boat ramps before dawn, hoping to catch their limit of these fish. Note: April has historically marked the beginning of the fishing season for Coho ("Silver") salmon (Oncorhynchus kisutch), which range from Alaska to Monterey Bay, but are threatened in the southern end of their range. However, populations of these fish have declined so dramatically off California that both the recreational and commercial fisheries have been closed. Deep-sea midwater animals feed on "table scraps" from the spring bloom Marine snow--it's what's for dinner in the deep sea Following many spring upwelling events and diatom blooms, a blizzard of organic debris (known as "marine snow") sinks down from the surface waters into the deep sea. This marine snow supports an amazing variety of animals both on the seafloor and in the "midwater" (between the surface and the seafloor), as well as marine bacteria and other microbes. At any given time, the amount of marine snow sinking down through the water is directly related to the amount of algae growing and being consumed in the surface waters. Thus, each spring diatom bloom is typically followed by a pulse of marine snow. Looking out the port of a deep-sea submersible in Monterey Bay during spring is like driving through a snow storm. But instead of frozen water, the "snow" is bits of organic mater. As a broad generalization, marine snow consists of poop, snot, and dead bodies. During the spring months, most of the "poop" in marine snow consists of tiny, sausage-like fecal pellets from copepods and krill. The "snot" consists of mucus that is secreted by diatoms, or by animals that use mucus nets to feed on marine snow (some of which are described below). The "dead bodies" in marine snow are those of diatoms, copepods, krill larvae, and other tiny marine animals that are constantly eating and being eaten in the surface waters. Note: Even when they are alive, copepods and krill provide a regular supply of "body parts" to the deep sea. Like all crustaceans, copepods and krill have hard outer shells. In order to grow, these animals must shed their outer shells. Thus, each time these animals transition from one larval stage to another or experience a growth spurt (for example, after gorging on spring-bloom diatoms), their shells split apart and are jettisoned, sinking down toward the seafloor. Although the components of marine snow don't sound very nutritious (or appetizing, for that matter), they are the primary source of food for many animals in the deep sea. Since there is no sunlight in deep water, no algae can grow, so marine snow takes the place of living algae as a primary source of food for many deep-sea animals. Animals that collect marine snow and other particles from the water are called "detritus feeders." After a "detritus feeder" has sucked the nutrients out of a bit of marine snow, it releases its own excrement. This excrement, in turn, feeds other animals. Thus, a single "flake" of marine snow may be consumed several times before it reaches the seafloor. Each time the marine snow is eaten and pooped, a bit more of the nutritious organic material is used up, so the deeper you go, the less nutrition is available to detritus feeders. Note: Marine bacteria also do their part to decompose marine snow, and are an important part of many deep-sea food webs. In fact, many animals that eat marine snow get as much nutrition from bacteria living on marine snow particles as from the marine snow itself. Larvaceans eat marine snow  Larvaceans use mucus filters such as this one to strain tiny food particles from the surrounding seawater. (Source: NOAA Office of Ocean Exploration) Many midwater animals use filters to capture bits of falling marine snow. Animals known as larvaceans are some of the most common midwater filter feeders in Monterey Bay during spring. Larvaceans drift in the Bay's waters at all times of year, but become particularly abundant from April through June. Larvaceans are small, tadpole-like creatures, usually less than half an inch long, that create mucus filters up to three feet across. The larvacean lives inside its mucus filter (which marine biologists call its "house"). It wiggles it's tail to pump water through the house and keep the filter inflated like a balloon. A larvacean's house strains out particles of marine snow that are too large for the larvacean to eat. The larvacean uses a second, smaller filter to capture the microscopic particles of marine snow that are its food. Following spring diatom blooms, a larvacean's house becomes clogged with marine snow after less than a day's use. When this happens, the larvacean simply swims out of its house and secretes another one. Note: In the weightless, borderless midwater environment, larvacean houses form "hard" surfaces that are colonized by a variety of organisms. Each larvacean house is like a miniature planet that supports a thriving ecosystem of copepods, isopods, protozoans, and bacteria. Poeobius worms eat marine snow In addition to larvaceans, another detritus eater that benefits from the spring blooms is a small, pudgy worm called Poeobius meseres. Poeobius worms drift in the dark, still waters 1,000 to 3,500 feet below the ocean surface, and feed on marine snow that they trap using a mucus web and small hairy tentacles. Because Poeobius worms have clear bodies, it is relatively easy for a marine biologist to see what an individual worm ate for its most recent meal. During March and April, Poeobius worms in Monterey Bay eat mostly the fecal pellets and molted skins of krill larvae. These worms will also eat diatoms, but few diatoms arrive intact below 1,000 feet, since most are eaten before they get to this depth. Seafloor animals benefit from seasonal pulses of marine snow Directly or indirectly, almost all of the animals on the deep seafloor depend on marine snow for food. Many seafloor animals, such as worms, sea cucumbers, crabs, and shrimp, feed directly on marine snow that has settled to the seafloor. However, by the time marine snow reaches the deep sea, it contains very little in the way of nutrients. Thus, most deep-sea animals have to eat a lot of marine snow in order to get enough food to survive. If there is any time when food is abundant in the deep sea (at least those deep-sea areas within a hundred miles of the shore), it is following the spring diatom blooms. Following strong upwelling events and intense diatom blooms, marine snow arrives on the seafloor ten times faster than at other times of year. In Monterey Bay, the biggest pulses of marine snow typically reach the seafloor between March and June. One study found that the amount of food delivered by each "marine snow storm" depended on the strength of upwelling and the productivity of diatoms that bloomed during the previous two weeks. Other studies have shown that even seafloor animals farther from shore, in water 13,000 feet deep, are effected by spring upwelling along the coast. English sole and halibut move toward shore to spawn Perhaps in response to the increased food available to deep-sea animals in spring, several common seafloor predators (and favored seafood items) mate in spring. These include English sole, halibut, and Dungeness crab. Beginning in February, English sole move from deep water toward the middle of the continental shelf. During March or April, where the water is 150 to 250 feet deep. Their spring migration to shallower water puts the sole within reach of hungry harbor seals, which feed on sole only at this time of year. Halibut also migrate toward shore in spring, perhaps responding to increasing populations of anchovies and market squid, as well as worms and shrimp on the seafloor at this time of year. They will remain close to shore through fall. Adult Dungeness crabs mate  Dungeness crabs mating. If you look closely, you can see that this photo shows not one but two crabs. The smaller female crab is underneath the male, on her back, with just a few of her claws showing. (Source: Scott Groth/Oregon Department of Fish and Wildlife) Adult Dungeness crabs may also take advantage of the increase in food on the seafloor in spring. They need this extra nutrition to carry themselves through the mating season, which runs from March through May in the Central Coast area. Note: Along the Oregon coast, Dungeness crabs mate later, in summer or fall. Dungeness crabs are scavengers and opportunistic predators that often lie in wait, partially buried in seafloor mud, with only their eyes showing. They eat all sorts of bottom-dwelling animals, including fish, shrimp, clams, worms, isopods, and amphipods, and especially others of their own kind. As one researcher put it, “Cannibalism is prevalent among all age groups." Mating is an arduous process for Dungeness crabs. It begins when the shell of the female crabs becomes soft, in preparation for their annual molt. At this point, the female crabs release a chemical into the seawater that attracts male crabs. When a male crab finds a female ready to molt, he "sweeps her off her feet," holds her in his claws, and presses her belly against his. He then proceeds to carry the female around in this embrace for the next week, perhaps to prevent other males from mating with her. After seven days of being carried around, the female realizes that she is about to molt, and struggles to escape. The male lets her go. But as soon as the female gets back on her feet and sheds her shell, the male inserts his copulatory pleopods into her seminal receptacles. The crabs then mate for anywhere from 30 minutes to two hours. After mating, the male crab carries the female around for another day or two. Then they go their separate ways. The female crab, however, still has a lot of work to do. She will store the male's sperm in her body until October or November, when she releases millions eggs and then carries them on her stomach for several months. |
